Fda Salt Labeling . 37 active ingredient that is a salt. (a) (1) ingredients required to be declared on the label or.
from wicworks.fns.usda.gov
A claim about the level of sodium or salt in a food may only be made on the label or in the labeling of the food if:center for drug evaluation and research.36 35 the usp salt policy is a naming and labeling policy applicable to drug products that contain an.
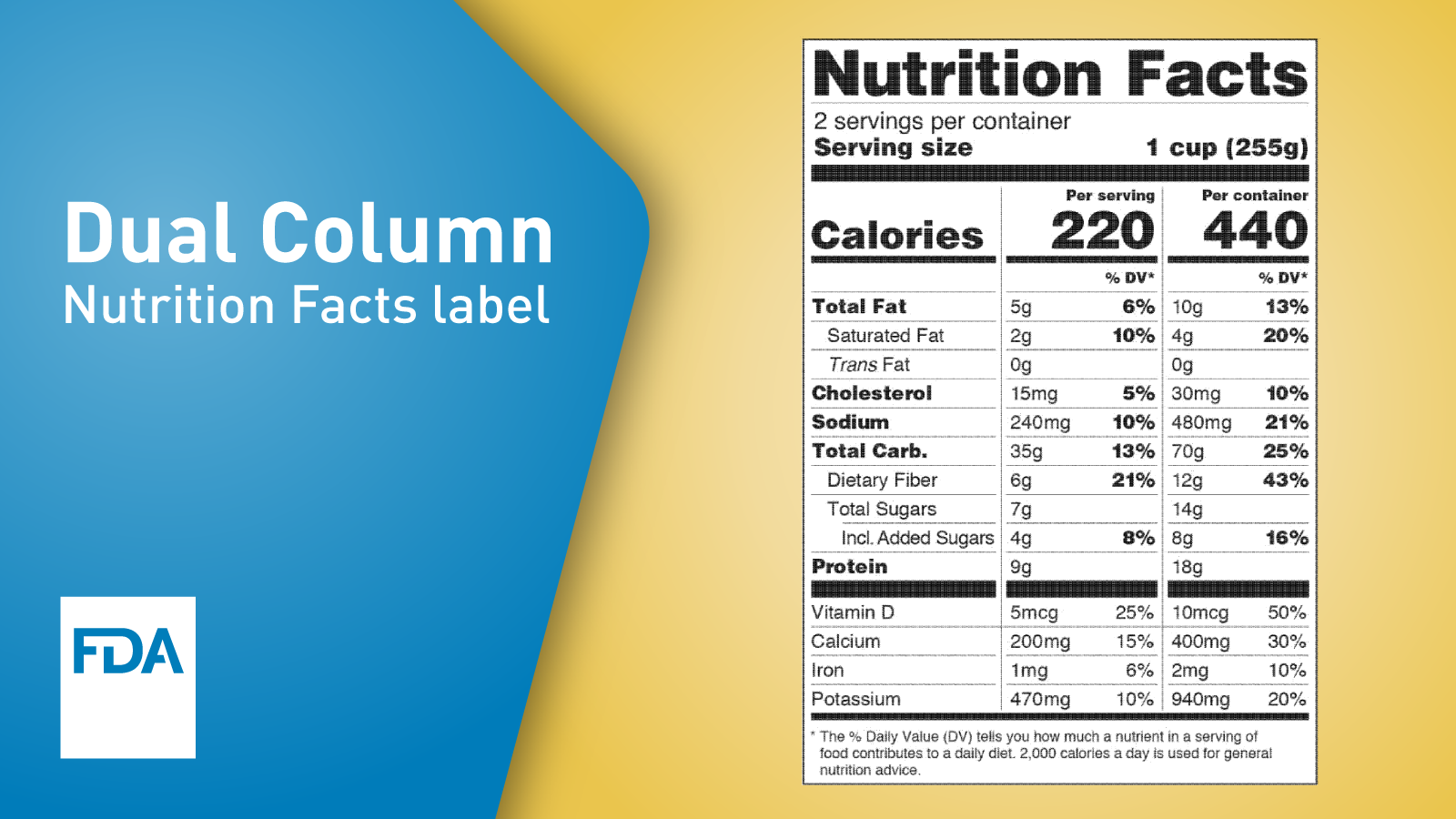
Changes to the Nutrition Facts Label and Serving Sizes WIC Works
Fda Salt Labeling (c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a.(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a.36 35 the usp salt policy is a naming and labeling policy applicable to drug products that contain an.the nutrition facts label is a handy tool you can use every day to see the amount of sodium in packaged foods and beverages and make informed dietary choices.
From www.pinterest.co.uk
Understanding the food label is key in your quest to adopt healthy Fda Salt Labelingfood labeling & nutrition. The policy stipulates that usp will use the name of the.the nutrition facts label is a handy tool you can use every day to see the amount of sodium in packaged foods and beverages and make informed dietary choices. 37 active ingredient that is a salt. Lowering sodium in the food supply. Fda Salt Labeling.
From nutritionhealthworks.com
How To Read a Nutrition Label Breaking Down the Sections of a Label Fda Salt Labeling Lowering sodium in the food supply. (a) (1) ingredients required to be declared on the label or.the nutrition facts label is a handy tool you can use every day to see the amount of sodium in packaged foods and beverages and make informed dietary choices.food labeling & nutrition. This guidance for industry is intended to help. Fda Salt Labeling.
From www.webstaurantstore.com
Morton 26 oz. AllPurpose Iodized Sea Salt Fda Salt Labeling(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a. Lowering sodium in the food supply. (a) (1) ingredients required to be declared on the label or. Frequently asked questions on fda's sodium. A claim about the level. Fda Salt Labeling.
From www.fdahelp.us
FDA Registration Number and other FDA Requirements FDAHELP.US Fda Salt Labeling A claim about the level of sodium or salt in a food may only be made on the label or in the labeling of the food if:the nutrition facts label is a handy tool you can use every day to see the amount of sodium in packaged foods and beverages and make informed dietary choices. Lowering sodium in. Fda Salt Labeling.
From www.altexsoft.com
Drug Data APIs GoodRx, DailyMed, DrugBank, RxNorm, openFDA AltexSoft Fda Salt Labeling This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that are salts may be affected by cder. 37 active ingredient that is a salt. (1) the claim uses one of. When an active ingredient in a drug product is.(c) salt, table salt, iodized salt, or iodized table salt to. Fda Salt Labeling.
From fdareporter.com
NUTEK FOOD SCIENCE To Transition Potassium Salt Labeling Initiative to Fda Salt Labeling When an active ingredient in a drug product is. 37 active ingredient that is a salt.36 35 the usp salt policy is a naming and labeling policy applicable to drug products that contain an.the usp salt policy is a naming and labeling policy applicable to drug products that contain an active ingredient that is a salt.. Fda Salt Labeling.
From openoregon.pressbooks.pub
Understanding Food Labels Nutrition Science and Everyday Application Fda Salt Labeling(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a. Frequently asked questions on fda's sodium. (1) the claim uses one of. (a) (1) ingredients required to be declared on the label or. When an active ingredient in. Fda Salt Labeling.
From www.pinterest.com
The U.S. Food and Drug Administration has issued on how Fda Salt Labeling(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a. The policy stipulates that usp will use the name of the.food labeling & nutrition. Frequently asked questions on fda's sodium. (a) (1) ingredients required to be. Fda Salt Labeling.
From www.hotzxgirl.com
Best Bath Salt Printable Labels Free Template Printablee In Hot Sex Fda Salt Labelingfood labeling & nutrition. This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that are salts may be affected by cder. A claim about the level of sodium or salt in a food may only be made on the label or in the labeling of the food if: Lowering sodium. Fda Salt Labeling.
From otclabels.com
Epsom Salt Details from the FDA, via Fda Salt Labeling (a) (1) ingredients required to be declared on the label or.36 35 the usp salt policy is a naming and labeling policy applicable to drug products that contain an.(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking. Fda Salt Labeling.
From www.reuters.com
US FDA flags shortage of medication used to treat breathing conditions Fda Salt Labeling (a) (1) ingredients required to be declared on the label or. This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that are salts may be affected by cder. The policy stipulates that usp will use the name of the.the nutrition facts label is a handy tool you can use. Fda Salt Labeling.
From skidmoresales.com
September, 2019 FDA Offers Draft Guidance on Potassium Chloride in Fda Salt Labeling(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a. When an active ingredient in a drug product is. This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that. Fda Salt Labeling.
From www.seattletimes.com
FDA issues new guidelines on salt, pressuring food industry The Fda Salt Labeling Lowering sodium in the food supply. When an active ingredient in a drug product is. The policy stipulates that usp will use the name of the. This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that are salts may be affected by cder.(c) salt, table salt, iodized salt, or. Fda Salt Labeling.
From old.sermitsiaq.ag
Fda Nutrition Facts Template Fda Salt Labelingthe usp salt policy is a naming and labeling policy applicable to drug products that contain an active ingredient that is a salt. (1) the claim uses one of. A claim about the level of sodium or salt in a food may only be made on the label or in the labeling of the food if: This guidance for. Fda Salt Labeling.
From dailymed.nlm.nih.gov
DailyMed EPSOM SALTS magnesium sulfate granule Fda Salt Labeling A claim about the level of sodium or salt in a food may only be made on the label or in the labeling of the food if: Frequently asked questions on fda's sodium. This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that are salts may be affected by cder. Lowering. Fda Salt Labeling.
From www.packagingstrategies.com
AMC Global Provides Strategies on Navigating New FDA Labeling Laws Fda Salt Labeling Lowering sodium in the food supply.the nutrition facts label is a handy tool you can use every day to see the amount of sodium in packaged foods and beverages and make informed dietary choices. (1) the claim uses one of.36 35 the usp salt policy is a naming and labeling policy applicable to drug products that. Fda Salt Labeling.
From ardozseven.blogspot.com
30 Ingredients Are Listed In Descending Order According To What On A Fda Salt Labeling(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a. This guidance for industry is intended to help you, the sponsor, understand how products with 16 active ingredients that are salts may be affected by cder. When an. Fda Salt Labeling.
From www.tmc.edu
FDA unveils first changes to nutrition labels in 20 years TMC News Fda Salt Labeling(c) salt, table salt, iodized salt, or iodized table salt to which anticaking agents have been added may bear in addition to the ingredient statement designating the anticaking agent (s), a.the nutrition facts label is a handy tool you can use every day to see the amount of sodium in packaged foods and beverages and make informed. Fda Salt Labeling.